The prevalence of severe congenital heart disease (CHD) in adults increased by 85% from 1985 to 2000, significantly outpacing the increase in prevalence in the pediatric population. Likewise, the median age of patients with severe CHD increased from 11 years in 1985 to 17 years in 2000. Although improved survival into adulthood for patients with CHD can be attributed to improved surgical techniques, percutaneous techniques, understanding of multisystem effects of CHD, and medical management, the fact that the median age of the total CHD population in 2002 was 40 years and that of the severe CHD population was 29 years indicates that survival is not normal in this cohort. Although mortality rates for adults with CHD in the 18- to 64-year age group have decreased commensurate with decreases for the general population, the median age of death in one population-based study was 23 years for patients with severe CHD and 48.8 years for all CHDs in another large cohort. Seventy-seven percent of deaths in this cohort were attributed to cardiovascular causes, with 26% of deaths attributable to chronic heart failure and 19% to sudden death. Given that health care utilization rates are highest in the final year of life and that chronic issues were the cause of death for many patients with CHD, the higher mortality and younger median age of death have significant implications for health care utilization by this population. In 2 large cohorts, hospitalization rates for adults with CHD were 50%, twice that of the general population. Sixty-one percent of admissions were for cardiovascular indications, with arrhythmias being the most common cardiovascular cause of admissions (31%).
As survival to adulthood increases for patients with CHD, so does the number of adult congenital patients who will require intensive care management. Sixteen percent of adults with CHD in the Quebec cohort required intensive care unit (ICU) admission, and ICU length of stay was significantly higher for patients with severe CHD (relative risk, 2.12; 95% confidence interval, 1.80-2.50). At Children’s Hospital Boston, the total number of adults with CHD admitted to the cardiac intensive care unit (CICU) has increased more than 3-fold between 1995 and 2009, representing an increase from 3.6% to 9.1% of all admissions (Fig 1).
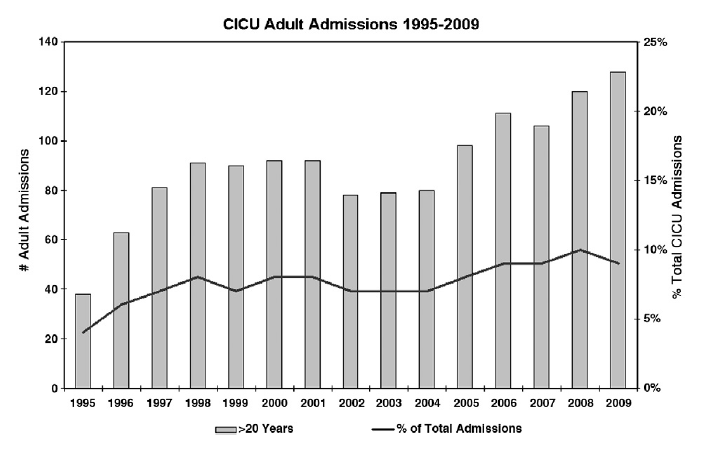
Fig 1. Number of adults with CHD admitted to the pediatric CICU as total number and percentage of all ICU admissions, 1995-2009.
General considerations
Location of care, composition of the ICU team, and multisystem nature of disease
Because of the palliative rather than curative nature of most surgeries for CHD, adult congenital patients may present to the ICU setting either in the perioperative period or for management of complex medical disease. Exceptional care of these patients requires the recognition that the sequelae of years of perturbed cardiovascular physiology involve multiple organ systems and that collaborative multispecialty care by physicians and other practitioners with specific training in the management of adults with CHD patients is essential. In addition to adult congenital cardiologists and cardiothoracic surgeons with extensive congenital cardiac surgical expertise, the team caring for adult congenital patients in the ICU needs to include intensivists who are facile with the pathophysiology of CHD, electrophysiologists, and consultants from multiple subspecialties including nephrology, gastroenterology and hepatology, hematology, neurology, psychiatry, and social work. The optimal location to care for the adult with CHD requiring ICU care is a matter of debate; nursing and physician providers in a pediatric ICU setting may be less facile and comfortable with adult intensive care management, whereas adult ICU providers may lack expertise in the unique cardiopulmonary physiology of the adult with CHD. Excellent outcomes have been achieved for adults with CHD undergoing surgery in pediatric centers and some data suggest lower mortality for adults with CHD who undergo surgery at pediatric centers by trained congenital heart surgeons compared with those who undergo operations at general medical facilities by either congenital heart or general cardiac surgeons.
At Children’s Hospital Boston, the percentage of adults with CHD admitted to the CICU following cardiac surgical procedures has steadily increased relative to admissions for medical management since 2004 (Fig 2). Increase in surgical admissions is reflective of the fact that even lesions that undergo “full anatomical repairs,” such as tetralogy of Fallot or transposition of the great arteries, frequently require reoperation, with one large series reporting nearly half of all CHD patients requiring more than one operation. Roughly half of the adults with CHD who present for surgery require primary repair, and half require reoperation. Although reported surgical mortality is low for adults with CHD (1.5%- 7%), as many as 24% of patients experience severe postoperative complications, including arrhythmias, renal failure, stroke, and multiorgan system failure. The risk of death and severe complications in the perioperative period is substantially higher for patients with significant cyanosis or with highly complex disease. Careful preoperative multisystem assessment of adults with CHD is critical to reduce the risk of severe complications and death. Preoperative evaluation should include careful evaluation of cardiac anatomy and function; assessment of renal (nuclear glomerular filtration rate [GFR] or creatinine clearance), pulmonary, and hepatic function; as well as assessment of hematologic parameters and modifiable cardiac risk factors (hypertension, hypercholesterolemia, diabetes).
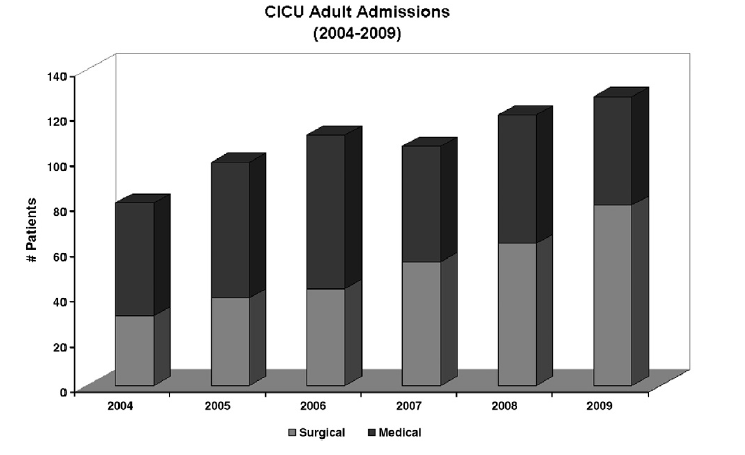
Fig 2. Number of surgical and medical admissions of adults with CHD to the pediatric CICU, 2004-2009.
Specific considerations for ICU care of the adult with CHD
Renal function and renal protection
Patients with CHD have increased risk of renal dysfunction that may start as early as childhood depending on complexity of lesions, degree of cyanosis, and number of surgical interventions. In a recent review of 1100 adults with CHD, 41% had mildly impaired GFR and 9% had moderate or severely impaired GFR, associated with a 3-fold increase in risk of mortality. Patients with cyanotic CHD, and in particular those with Eisenmenger syndrome, are more likely to have moderate to severely impaired renal function. Recognition of preexisting renal insufficiency in adults with CHD and proactive measures to protect renal perfusion and kidney function are essential in the ICU setting. Underlying renal dysfunction may be exacerbated by changes associated with cardiopulmonary bypass (decreased renal blood flow, changes in renin-angiotensin-aldosterone production, fluid retention, and third spacing) or by hemodynamic deterioration in the nonsurgical adult CHD patient, including the impact of arrhythmias. Formal evaluation of renal function before planned surgical interventions by determination of either nuclear GFR or creatinine clearance is necessary and appropriate in this patient population to assess perioperative risk for renal dysfunction and to plan renal protective strategies. Renal protective strategies include careful management of hemodynamics and renal perfusion pressure; minimization of exposure to nephrotoxic agents, including medications and intravenous contrast agents; and pharmacologic interventions to optimize renal perfusion and minimize nephrotoxicity of contrast agents.
Renal perfusion pressure, defined as the difference between mean arterial pressure and central venous pressure, is often decreased in the adult with CHD in the ICU setting on the basis of increased central venous pressures, particularly in patients with significant right heart disease, such as those with tetralogy of Fallot or Ebstein anomaly of the tricuspid valve, or in those with single-ventricle physiology palliated to a Fontan circulation. Judicious hemodynamic monitoring, including the use of central venous pressure monitoring catheters, coupled with the use of inotropes and vasoactive medications to maintain mean arterial pressure while avoiding renal arterial constriction, is an essential step toward renal protection through maintenance of renal perfusion pressures.
Renal perfusion pressure may also be adversely affected through increased intraabdominal pressure, such as that seen in patients with right heart failure and significant ascites. Intraabdominal hypertension reduces the renal filtration gradient, leading to oliguria and renal failure. Patients with a Fontan circulation or right heart failure and increased central venous pressures may be particularly susceptible to the deleterious effects of severe ascites. Decreased diaphragmatic excursion in this setting leads to increased need for positive pressure ventilation that in turn raises Fontan or right heart pressures and may decrease pulmonary venous return to the heart resulting in decreased cardiac output, which in turn further decreases renal perfusion. In the general ICU setting, measurement of bladder pressures to monitor and guide therapy for intraabdominal hypertension has improved outcomes in the setting of abdominal compartment syndrome. Although no specific data exist for the adult CHD population, similar intraabdominal pressure monitoring may also be useful in the adult CHD population. Certainly, controlled drainage of ascites in the setting of clinical abdominal compartment syndrome may improve renal perfusion in the adult with CHD in the ICU setting, although caution must be exercised to avoid sudden a drop in preload and the resultant reduction in cardiac output.
Pharmacologic strategies may also be useful to optimize renal perfusion. Fenoldopam, a dopamine 1 receptor specific agonist that causes arterial and arteriolar vasodilation, has been studied in general adult cardiac populations as a renal protective agent on the basis of its ability to increase renal blood flow both in the setting of cardiac surgery and around the time of contrast administration. Meta-analysis of 13 randomized and case-matched studies of fenoldopam administration during cardiopulmonary bypass in a standard (nonCHD) adult population demonstrated reduced need for renal replacement therapy, decreased in-hospital death, and shorter duration of ICU stay. Although no specific data exist on the prophylactic use of fenoldopam in the adult CHD population undergoing cardiac surgery, this may be a reasonable strategy for renal protection particularly in those patients with preexisting renal insufficiency.
The role of fenoldopam and other putative renal protective agents, including n-acetylcysteine (NAC) and sodium bicarbonate, in the prevention of contrast-induced nephropathy is controversial but may still warrant consideration. A multicenter, randomized, placebo-controlled trial of fenoldopam around angiography in adult non-CHD patients with mildly to moderately decreased GFR showed no decrease in contrast-induced nephropathy. NAC, an antioxidant that scavenges oxygen free radicals and may thereby prevent oxidative tissue damage related to contrast administration, and sodium bicarbonate show more promise as renal protective agents in this setting. Several studies, including randomized controlled trials, have demonstrated reduced incidence of contrast-induced nephropathy, defined as increase in creatinine by 0.5 mg/dL, in patients who received NAC plus hydration with sodium bicarbonate vs hydration with isotonic sodium chloride solution alone. However, these studies failed to show a reduction in clinically important outcomes such as renal failure requiring dialysis. Based on these data, prehydration with sodium bicarbonate plus NAC is frequently used for the prevention of contrast-induced nephropathy and can be reasonably extended to the adult CHD population with baseline renal insufficiency before catheterization or other radiologic studies requiring contrast administration.
Hepatic dysfunction and right heart failure
Chronic elevations in central venous pressure, either from long-standing congestive heart failure or from elevated right-sided pressures, leads to chronic hepatic venous congestion that can progress to centrilobular necrosis and fibrous replacement of hepatocytes and ultimately cardiac cirrhosis. Patients with a Fontan circulation are particularly at risk for these sequelae, and over time, portal hypertension may develop, leading to esophageal varices and the attendant risk of life-threatening gastrointestinal bleeding. The extent of cirrhosis and risk of varices are dependent on the length of time since the Fontan operation.
Acute hepatic dysfunction may be superimposed on chronic dysfunction during periods of low cardiac output or hypoxia. In this setting, marked elevation of transaminases may be seen in addition to changes in synthetic function.
Hematologic changes and coagulation
Hematologic changes corresponding with increased risk of both bleeding and clotting are seen in certain segments of the adult CHD population depending on underlying disease and physiologic state. Specifically, patients with long-standing cyanosis have polycythemia, though often with associated iron deficiency, and deficiency in platelet numbers and function. In addition, patients with underlying liver disease, such as those with right heart failure and in particular the Fontan circulation, may have abnormalities of hepatic synthetic function leading to prolonged prothrombin times and bleeding risk. The Fontan population in particular is at increased risk for thrombosis, likely related to multiple physical (stasis within the Fontan pathway) and biochemical (increased factor VIII levels) factors. Care of the adult with CHD in the ICU setting requires recognition of increased risk of both clotting and bleeding and attention to details such as deep venous thrombosis prophylaxis, early mobilization after surgery, and careful monitoring of coagulation status.
Pulmonary function and cardiopulmonary interactions
Patients with long-standing complex CHD may have compromised pulmonary function related to hypoplasia of one lung, restrictive lung disease, scoliosis, or diaphragmatic paralysis secondary to prior phrenic nerve injury. Additional risk factors such as history of cigarette smoking may further diminish pulmonary capacity. Diminished pulmonary reserve will be further compromised in patients who are significantly desaturated at baseline.
Respiratory support of the patient with complex CHD, in particular, the use of positive pressure ventilation, requires a sound understanding of baseline pulmonary status and underlying cardiac physiology but also an appreciation of the significant cardiopulmonary interactions that can be seen in the setting of complex CHD. Patients with primarily left-sided disease, including left ventricular (LV) dysfunction and aortic or mitral valve disease with ether low cardiac output or left atrial hypertension, may benefit substantially from positive pressure ventilation. LV wall stress, equivalent to the transmural pressure gradient, is the difference between intrathoracic pressure and LV systolic pressure. In the setting of positive pressure ventilation, intrathoracic pressure is increased. Hence, the transmural pressure gradient is decreased. LV wall stress and thus afterload are effectively reduced, which can lead to increased cardiac output. On the other hand, anything that decreases intrathoracic pressure such as increased work of breathing may negatively impact cardiac output.
The effects of positive pressure ventilation are substantially different in the patient with right heart failure, significant right atrioventricular valve regurgitation, pulmonary hypertension or, in particular, a Fontan circulation characterized by passive pulmonary blood flow. Right ventricular output is determined primarily by right ventricular preload, which is determined by the pressure gradient between the extrathoracic great veins and the right atrium. During spontaneous respiration, right atrial pressure falls during inspiration, thereby increasing the pressure gradient. During positive pressure inspiration, however, right atrial pressure increases, the great vein to right atrial pressure gradient decreases, and right ventricular preload decreases. The result is a decrease in right ventricular output. In patients with poor right ventricular function, a drop in right ventricular preload may significantly compromise right ventricular output, which leads to decreased LV preload and compromised systemic output. Systemic output may also be compromised because of ventriculo-ventricular interactions. Elevated right ventricular pressures in the setting of positive pressure ventilation leads to septal shift and may further reduce LV filling.
The effects of positive pressure ventilation are substantially different in the patient with right heart failure, significant right atrioventricular valve regurgitation, pulmonary hypertension or, in particular, a Fontan circulation characterized by passive pulmonary blood flow. Right ventricular output is determined primarily by right ventricular preload, which is determined by the pressure gradient between the extrathoracic great veins and the right atrium. During spontaneous respiration, right atrial pressure falls during inspiration, thereby increasing the pressure gradient. During positive pressure inspiration, however, right atrial pressure increases, the great vein to right atrial pressure gradient decreases, and right ventricular preload decreases. The result is a decrease in right ventricular output. In patients with poor right ventricular function, a drop in right ventricular preload may significantly compromise right ventricular output, which leads to decreased LV preload and compromised systemic output. Systemic output may also be compromised because of ventriculo-ventricular interactions. Elevated right ventricular pressures in the setting of positive pressure ventilation leads to septal shift and may further reduce LV filling.
Arrhythmias
Arrhythmias are a significant cause of morbidity and mortality in adults with CHD and occur with increasing frequency with age. Upwards of 50% of patients with complex CHD will develop significant arrhythmias over their lifetime. Arrhythmias are the leading cause of hospital admission for adults with CHD, are a significant predictor of mortality, and complicate the postoperative period in as many as 30% of adults with CHD undergoing cardiac surgery. Arrhythmias are a frequent primary indication for admission of the adults with CHD to the ICU. For patients with marginal systemic ventricular function or single-ventricle physiology, loss of atrioventricular synchrony and poor rate control may substantially impair cardiac output and lead to a cycle of low output requiring increased inotropic support that may further drive tachyarrhythmias. Close collaboration with electrophysiologists comfortable with the pathophysiology and management of CHD is required for these patients.
Resuscitation and mechanical support
Successful resuscitation of the adult with CHD from cardiopulmonary arrest represents a significant challenge because of the potential for preexisting multiorgan system dysfunction and the complex cardiopulmonary physiology. Patients with significant atrioventricular valve regurgitation, pulmonary hypertension, and Fontan circulation are likely to have a worse outcome from cardiopulmonary resuscitation (CPR) given the difficulty of maintaining LV ejection even in the face of excellent chest compressions. Passive pulmonary blood flow in the Fontan circulation will take place only during the recoil phase of chest compressions, so allowing complete recoil is critical in this group of patients. No data on the utility of extracorporeal membrane oxygenation to aid CPR in adults with CHD are available. Review of the Extracorporeal Life Support Organization registry data has demonstrated overall survival following cannulation for extracorporeal membrane oxygenation (ECMO) during CPR of 25% in the general adult population compared with 51% in the general pediatric population. However, these survival rates may be overly optimistic in the complex adult CHD population. Extracorporeal membrane oxygenation to support the adult with CHD not in the setting of CPR may be more successful, although this too is fraught with problems. Multiple vascular occlusions can be common in this population given multiple prior interventions, adding significant difficulty and complexity to cannulation of the peripheral vasculature. Lower extremity ischemia and venous congestion related to the vascular compromise from large bore cannulas required to adequately support an adult patient are not uncommon.39 Excessive bleeding may ensue when extracorporeal membrane oxygenation is used for postcardiotomy support.
Similarly, limited data are available on the use of ventricular assist devices (VADs) to support adult patients with complex CHD. The mechanics of VAD support in patients with complex CHD, particularly palliated singleventricle patients, may be more complicated than those in the general adult population given variable and unusual anatomy. Although successful VAD support as bridge to recovery or bridge to transplantation has been reported in several patients with superior cavopulmonary anastomosis (eg, bidirectional Glenn) or Fontan circulation, adequate filling of a functionally left-sided VAD is dependent on passive pulmonary blood flow. Rightsided support to aid filling of a left-sided device is technically challenging given the lack a right-sided capacitance chamber in the Fontan circulation.
Ref: Allan CK, Intensive care of the adult patient with congenital heart disease, Prog Cardiovasc Dis. 2011 Jan-Feb;53(4):274-80.
