In this section we review one of the ICU management Protocols.
ADMISSION, DISCHARGE CRITERIA AND TRIAGE
ADMISSION CRITERIA
The Intensive Care Unit is an expensive resource area and should be reserved for patients with reversible medical conditions with a reasonable prospect of substantial recovery.
Patients with the following conditions are candidates for admission to the General Intensive Care Unit. The following conditions include, but are not limited to:
Respiratory
-
Acute respiratory failure requiring ventilatory support
-
Acute pulmonary embolism with haemodynamic instability
-
Massive haemoptysis
-
Upper airway obstruction
Cardiovascular
-
Shock states
-
Life-threatening dysrhythmias
-
Dissecting aortic aneurysms
-
Hypertensive emergencies
-
Need for continuous invasive monitoring of cardiovascular system (arterial pressure, central venous pressure, cardiac output)
Neurological
-
Severe head trauma
-
Status epilepticus
-
Meningitis with altered mental status or respiratory compromise
-
Acutely altered sensorium with the potential for airway compromise
-
Progressive neuromuscular dysfunction requiring respiratory support and / or cardiovascular monitoring (myasthenia gravis, Gullain- Barre syndrome)
-
Brain dead or potentially brain dead patients who are being aggressively managed while determining organ donation status
Renal
-
Requirement for acute renal replacement therapies in an unstable patient
-
Acute rhabdomyolysis with renal insufficiency
Endocrine
-
Diabetic ketoacidosis complicated by haemodynamic instability, altered mental status
-
Severe metabolic acidotic states
-
Thyroid storm or myxedema coma with haemodynamic instability
-
Hyperosmolar state with coma and/or haemodynamic instability
-
Adrenal crises with haemodynamic instability
-
Other severe electrolyte abnormalities, such as:
-
-
Hypo or hyperkalemia with dysrhythmias or muscular weakness
-
Severe hypo or hypernatremia with seizures, altered mental status
-
Severe hypercalcemia with altered mental status, requiring haemodynamic monitoring
-
Gastrointestinal
-
Life threatening gastrointestinal bleeding
-
Acute hepatic failure leading to coma, haemodynamic instability
-
Severe acute pancreatitis
Haematology
-
Severe coagulopathy and/or bleeding diasthesis
-
Severe anemia resulting in haemodynamic and/or respiratory compromise
-
Severe complications of sickle cell crisis
-
Haematological malignancies with multi-organ failure
Obstetric
-
Medical conditions complicating pregnancy
-
Severe pregnancy induced hypertension/eclampsia
-
Obstetric haemorrhage
-
Amniotic fluid embolism
Multi-system
-
Severe sepsis or septic shock
-
Multi-organ dysfunction syndrome
-
Polytrauma
-
Dengue haemorrhagic fever/dengue shock syndrome
-
Drug overdose with potential acute decompensation of major organ systems
-
Environmental injuries (lightning, near drowning, hypo/hyperthermia)
-
Severe burns
Surgical
-
High risk patients in the peri-operative period
-
Post-operative patients requiring continuous haemodynamic monitoring/ ventilatory support, usually following:
-
vascular surgery
-
thoracic surgery
-
airway surgery
-
craniofacial surgery
-
major orthopaedic and spine surgery
-
general surgery with major blood loss/ fluid shift
-
neurosurgical procedures
-
Patients who are generally not appropriate for ICU admission
-
Irreversible brain damage
-
End stage cardiac, respiratory and liver disease with no options for transplant
-
Metastatic cancer unresponsive to chemotherapy and/or radiotherapy
-
Brain dead non-organ donors
-
Patients with non-traumatic coma leading to a persistent vegetative state
DISCHARGE CRITERIA
The status of patients admitted to an ICU should be reviewed continuously to identify patients who may no longer need ICU care. This includes:
-
When a patient’s physiologic status has stabilised and the need for ICU monitoring and care is no longer necessary
-
When a patient’s physiological status has deteriorated and / or become irreversible and active interventions are no longer beneficial, withdrawal of therapy should be carried out in the intensive care unit. Patient should only be discharged to the ward if bed is required.
Discharge will be based on the following criteria:
-
Stable haemodynamic parameters
-
Stable respiratory status (patient extubated with stable arterial blood gases) and airway patency
-
Oxygen requirements not more than 60%
-
Intravenous inotropic/ vasopressor support and vasodilators are no longer necessary. Patients on low dose inotropic support may be discharged earlier if ICU bed is required.
-
Cardiac dysrhythmias are controlled
-
Neurologic stability with control of seizures
-
Patients who require chronic mechanical ventilation (eg motor neuron disease, cervical spine injuries) with any of the acute critical problems reversed or resolved
-
Patients with tracheostomies who no longer require frequent suctioning
TRIAGE
Due to the limited number of ICU beds, triaging may be necessary.
The following factors will be taken into consideration in triaging:
-
Diagnosis
-
Severity of illness
-
Age and functional status
-
Co-morbid disease
-
Physiological reserve
-
Prognosis
-
Availability of suitable treatment
-
Response to treatment to date
-
Recent cardiopulmonary arrest
-
Anticipated quality of life
INVESTIGATIONS AND MICROBIOLOGICAL SURVEILLANCE
No routine investigations are to be done for patients admitted to the ICU
Basic investigations on admission
-
Full blood count (includes haemoglobin, total white and differential counts, platelet count)
-
Serum creatinine, blood urea and electrolytes (including Na+, K+, Cl-, Ca2+, Mg2+, Phosphate3-)
-
Liver function test
-
Prothrombin time (PT), activated partial thromboplastin time (APTT), coagulation screening
-
Arterial blood gas
-
Blood glucose level (hand held blood glucose analyzer is acceptable)
Additional tests on admission when indicated
-
Septic / microbiology screen as indicated
-
CXR (after placement of appropriate lines e.g. central venous line, nasogastric tube)
Patients requiring post-operative ventilation for a few hours may not require a routine CXR
-
ECG
Tests ordered daily
-
FBC: Hb, TWDC, platelet count
-
BUSE, creatinine
-
Other tests only when indicated
Microbiological Surveillance
-
MRSA screening (nasal swab only) on admission to ICU may be indicated in the following:
-
Patients who have been admitted for > 5 days in the ward
-
Patients with previous positive cultures for MRSA either in the blood, tracheal aspirate or urine
-
Patients admitted from other hospital
-
Patients admitted from long-term care institutions e.g. nursing homes
-
Patients on chronic renal dialysis
-
-
Tracheal aspirate for C&S
May be done once a week in intubated patients. (Note: not all positive cultures on routine surveillance are infective)
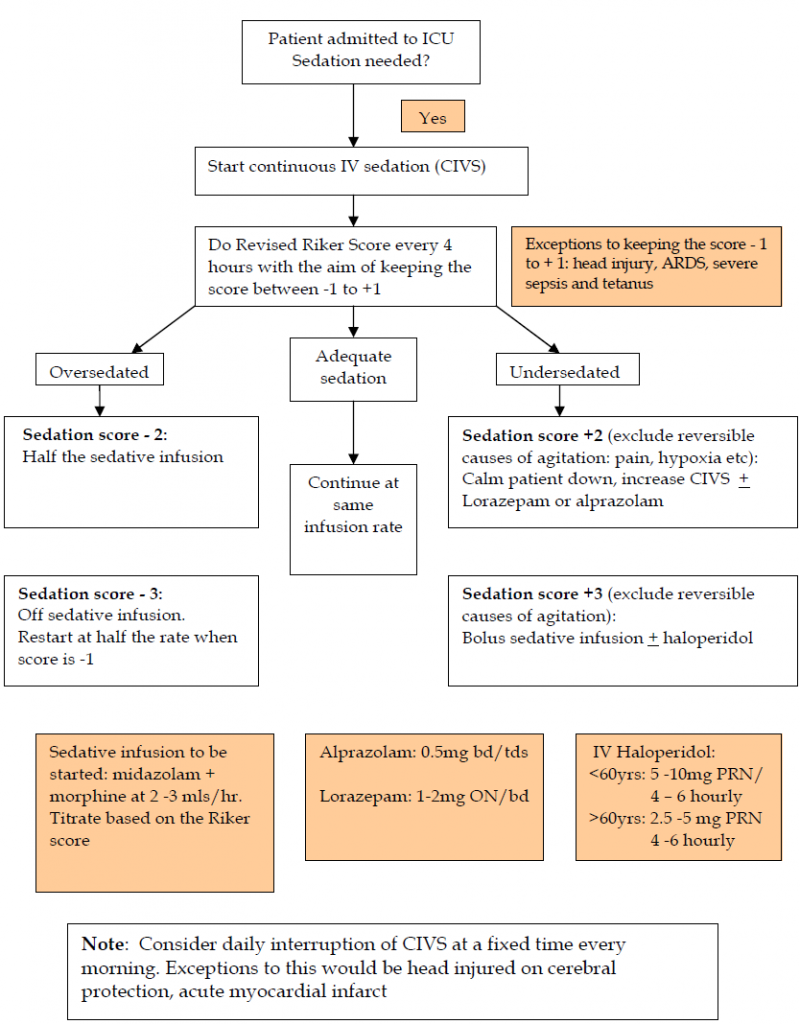
CONTINUOUS INTRAVENOUS SEDATION
Sedative medications are widely used in the intensive care unit. Titrating the dose of the medication based on a sedation scale will help prevent oversedation, treat undersedation as well as restlessness and agitation.
Nurses and doctors are encouraged to change the sedative infusion rate based on this protocol.
-
Patients are to be assessed for sedation and agitation based on the revised Riker Sedation and Agitation scale every 4 hours. The worst score within the last 4 hours is to be recorded.
-
Titrate the sedative infusion rate with the aim of keeping the sedation score between -1 to +1
-
Exceptions to keeping the sedation score between -1 and +1:
-
head injured on cerebral protection: sedation score -3
-
severe sepsis on high inotropic support: sedation score of at least -1
-
ARDS on high ventilatory support: sedation score of at least -2
-
tetanus: sedation score of at least -2
-
-
The standard sedative infusion to be used in patients admitted to ICU is midazolam and morphine. 30mg midazolam and 30mg morphine is diluted in up to 30mls normal saline. The infusion may be started between 2 – 3mls per hour
-
Fentanyl may be used instead of midazolam and morphine in the following conditions:
-
renal failure
-
hepatic failure
-
-
200mcg Fentanyl is diluted in up to 20mls normal saline. The infusion rate is between 2 – 5mls per hour (20 -50mcg/hour)
-
Postoperative cases that are for overnight ventilation may be put on
-
morphine + propofol
-
dexmedetomidine (only for 24 hours)
-
-
Consider daily interruption of continuous sedative infusion at a fixed time every morning.
-
If sedation score is +2, exclude other causes of agitation such as pain, hypoxia etc. Calm patient down by communicating with him. Increase the sedative infusion rate. There may be a need to add further sedation
For example: Tab. Lorazepam 1-2 mg ON / bd
Tab. Alprazolam 0.5mg bd / tds
-
If sedation score +3, exclude other causes of agitation. Bolus midazolam/morphine and increase infusion rate except in non-ventilated patients. IV haloperidol will probably be indicated:
Age < 60 years: 5 -10mg PRN / 4 -6hourly
Age > 60 years: 2.5 -5 mg PRN / 6 hourly
-
If sedation score: -2, half the intravenous sedative infusion. Decrease the sedative infusion every 4 hours until a score of -1 is achieved
-
If sedation score: -3, off sedative infusion. Assess 4 hours later. Restart at half the infusion rate once a score of -1 has been reached.
Patients that are paralysed need not be scored and should be denoted with a capital “P”
Revised Riker Sedation Agitation Scale
| Score | Description | Definition |
| +3 | Agitated and restless | When awaken or otherwise, pulling at ETT, trying to remove catheters or requires physical restraints |
| +2 | Awake but mildly agitated | Anxious but mildly agitated. Attempts to sit up but calms down with verbal instructions |
| +1 | Awake and calm | Awake, calm and easily follows commands |
| 0 | Aroused by voice and
remains calm |
Awakens easily to verbal stimuli. Remains awake, calm and easily follows command |
| -1 | Aroused by movement | Awakens to loud verbal stimuli or gentle shaking. Has eye contact for at least 10 seconds but drifts off to sleep OR Awakens to loud verbal stimuli or gentle shaking and follows simple commands |
| -2 | Aroused by painful stimuli | Localising or flexion to pain. Does not communicate or follow commands |
| -3 | Unarousable | Extension, minimal or no response to painful stimuli |
Algorithm for titration of continuous intravenous sedation using the Revised Riker Sedation Agitation Scale
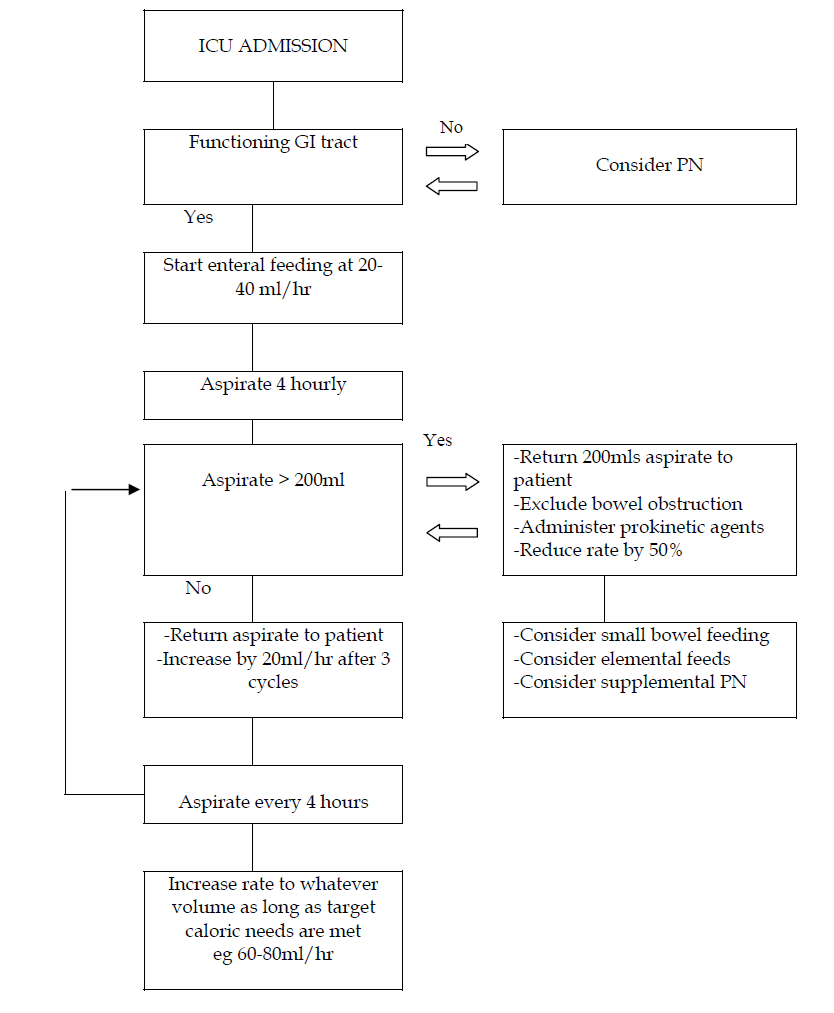
ENTERAL FEEDING
General Points
-
All ventilated patients must receive a nasogastric or orogastric tube. It is preferable to use 12FG in adults. The correct position of the tube should be confirmed by 2 out of the following 3 criteria. The criteria are (i) injecting 10-20 ml of air down the tube and auscultating the epigastric area (ii) use of litmus paper and (iii) radiography.
-
Early enteral feeding should be commenced within 24-48 hours after ICU admission whenever the gastrointestinal tract is deemed to be functioning. This applies to all mechanically ventilated patients who have been adequately resuscitated and hemodynamically stable.
-
Enteral feeding for patients who have undergone recent abdominal and bowel surgeries may require prior discussion with the surgeon and ICU specialist before commencement.
-
Patients should preferably receive feeding continuously during the acute phase. They can be switched to intermittent bolus technique later.
(i) Continuous feeding
-
Start at 20-40ml/hr continuously. Aspirate the feeding tube every 4 hours. If aspirate < 200ml, return all aspirate. Increase rate by 20ml/hr every 3 cycles till a flow rate that meets the caloric needs of the patient. Once target caloric needs are met, the feeds may be further diluted with water to meet the fluid requirements of the patient.
-
If aspirate >200ml, return 200ml aspirate to patient and reduce rate by 50% of initial rate. Exclude bowel obstruction first. If there is no clinical evidence of bowel obstruction, administer prokinetic agents (see 11). Once further aspirates are < 200ml, follow (i-a). If aspirates continue to exceed 200 ml after the above has been carried out, consider the use of small bowel feeding (see 10) and elemental formulas (see 9).
(ii) Intermittent bolus feeding
Start with 50ml every 3 hours. Aspirate before every feed.
-
If aspirate < 200ml return aspirate to patient. Increase by 50ml after every 4 feeds. Increase by 100 ml/feed every 24 hr till caloric needs are met. Once target caloric needs are met, the feeds may be further diluted with water to meet the fluid requirements of the patient.
-
If aspirate >200ml, return 200ml aspirate to patient and reduce by 50ml per feed. Exclude bowel obstruction first. If there is no clinical evidence of bowel obstruction, administer prokinetic agents (see 11). Once further aspirates are < 200ml, follow (ii-a). If aspirates continue to exceed 200 ml after the above has been carried out, consider the use of continuous feeding (see 4).
-
Where feasible, enteral feeds can be administered via the closed system. Closed system refers to the “ready to hang formulas”. Once connected the duration of feeding is 24 hrs. During initiation of feeding, use the open system (to reduce wastage) and where feasible, use the closed system once target feeding has been achieved.
-
All patients receiving feeding must be placed in the semi-recumbent position with the head of the bed elevated to 45 0. Head injured patients will have head elevated to 30 0. Patients who have to be prone should be in the anti-trendelenberg position (10-20 0)
-
Current recommendations suggest a target energy intake of 25 kcal/kg/day and at least 1.2-1.5 g/kg/day of protein. For obese patients, use 120% of ideal body weight (IBW) or (Actual Body Weight – IBW) X 0.25 + IBW. For underweight patients, use actual body weight. Energy intake should be adjusted according to the severity and type of illness. Feeding should be administered via a stepwise gradual introduction of feeds over first 48 hrs. Avoid overfeeding.
-
Use polymeric formulas (whole protein formula) for feeding.
-
Peptide based or elemental formulas (eg Peptamen, Alitrac) have been shown to be useful in patients with gastrointestinal complications (short bowel syndrome, pancreatitis). However, there is insufficient evidence to demonstrate any favorable treatment effects.
-
Small bowel feeding (nasojejunal/nasoduodenal) can be considered for patients who are intolerant to enteral feeding (high inotropic support, continuous infusion of sedatives, or paralytic agents or with high gastric residual volumes) or in pancreatitis
-
Motility agents such as IV metoclopramide 10-20mg 6-8 hourly and/or IV erythromycin 125 mg QID or 250 mg BID to be used in patients who experience feed intolerance (high gastric residuals, emesis).
-
Nutrient mixtures prepared in the kitchen (“blenderised diet”) should not be used as this type of feed is unbalanced, causes feeding tube occlusion and diarrhea secondary to bacterial contamination.
Types of enteral feeds
Type |
Energy |
Indications |
** Suggestedmaximumvolume/day |
Polymeric-Ensure-Enercal-Isocal-Nutren |
1Kcal/ml |
Most patients |
60-80 ml/hror200-250 ml 3 hourly |
-Glucerna-Nutren diabetic |
1Kcal/ml |
-Diabetic patients-Stress inducedhyperglycemia |
60-80 ml/hror200-250 ml 3 hourly |
Fiber-enriched-Jevity-Nutren fiber |
1Kcal/ml |
-Patient who developdiarrhea to non-fibercontaining feeds |
60-80 ml/hror200-250 ml 3 hourly |
Disease specific-Nepro |
2Kcal/ml |
-Renal failure with fluidrestriction and ondialysis |
30-40 ml/hror100-120 ml 3 hourly |
Disease specific-Suplena |
2Kcal/ml |
-Renal failure with fluid,protein, electrolytesrestriction and not ondialysis |
30-40 ml/hror100-120 ml 3 hourly |
Disease specific-Pulmocare |
1.5Kcal/ml |
-Patients withhypercapnia (eg COAD) |
50-60 ml /hror150-200 ml 3 hourly |
Elemental-Peptamen-Alitrac |
1Kcal/ml |
-Patients withgastrointestinalproblems (pancreatitis,short bowel syndrome) |
60-80 ml/hr |
** Please note that the suggested maximum volume is not restricted to the examples given. It is important that the target caloric needs are eventually met in whatever volume that is delivered to the patient. If the patient has increased fluid requirements, supplemental fluids may be given as diluted feeds (the maximum flow rates can be increased) or given intravenously
ALGORITHM FOR ENTERAL FEEDING (CONTINOUS METHOD)
INOTROPIC AND VASOPRESSOR SUPPORT
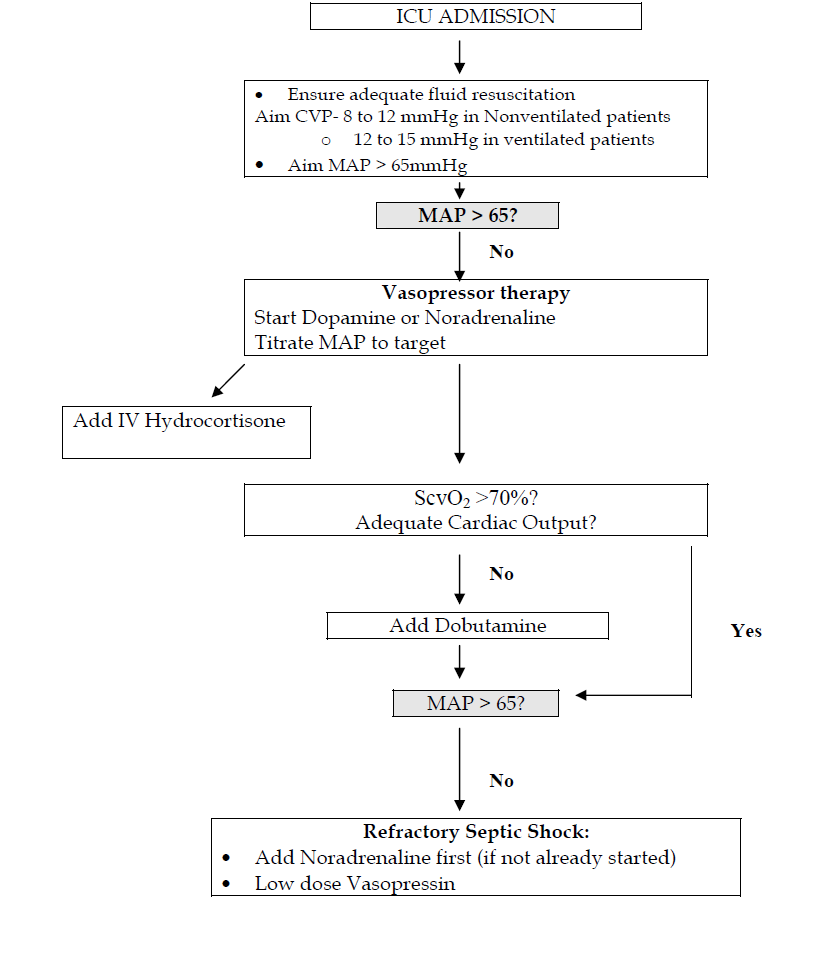
A. Inotropic and vasopressor support in Septic Shock
Septic shock is defined as hypotension despite adequate fluid resuscitation, together with evidence of perfusion abnormality (i.e. oliguria, mental impairment, lactic acidosis) in association with sepsis.
-
Ensure adequate fluid resuscitation.
-
Target CVP – for non ventilated patients is 8-12 mmHg and for ventilated patients is 12-15mmHg.
-
Target MAP > 65mmHg
The initial vasopressor of choice is either dopamine or noradrenaline, administered through a central venous catheter. Place arterial line as soon as practical.
-
Commence infusion dopamine (200mg diluted in 50mls 0.9% NS or D5%)
Dosage range 5-20mcg/kg/min
‘Dopamine-resistant’ shock is defined by MAP < 65 despite administrating dopamine at infusion dose of 20mcg/kg/min.
Or
Start infusion noradrenaline (4mg diluted in 50mls 0.9% NS or D5%)
Dosages range 0.02-1.5mcg/kg/min
-
Add IV hydrocortisone 50mg QID or 100mg TDS
-
Add infusion dobutamine (3-5mcg/kg/min) if ScvO2 <70%.
Consider dobutamine as inotropic support in patient with low cardiac output
(Infusion up to 20 mcg/kg/min)
Dilution: 250mg in 50mls 0.9% NS or D5%
Caution: dobutamine may drop MAP
4 If MAP ≤ 65mmHg despite adequate fluid resuscitation and high dose of vasopressors, may consider infusion vasopressin (20 Units diluted in 40mls 0.9%NS or D5%).
Dosages range 0.01-0.04 Units/min.
B. INOTROPIC AND VASOPRESSOR SUPPORT IN SEPTIC SHOCK
C. Inotropic and vasopressor support in Cardiogenic Shock
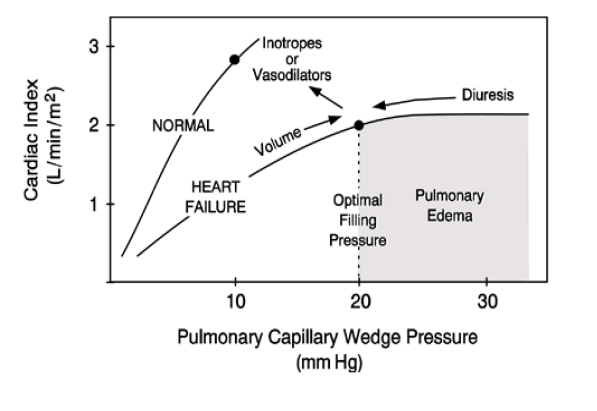
Dobutamine:
– an inodilator
– dilute 250mg in 50mls of 0.9% NS or D5%
– dosage range 5-20mcg/kg/min
– to be used when CI↓ (<2.2 l/mim/m²), MAP↓ (<65mmHg) with a high SVR (>2400 dyne.sec/cm5 m²
May need to start noradrenaline infusion if MAP drop (<60mmHg) while on an inodilator.
Consider adding infusion adrenaline (3mg in 50mls of 0.9%NS or D5%) at 0.02-1.0 mcg/kg/min.
It is desirable that use of inotropes is guided by cardiac output monitoring.
If remains in cardiogenic shock-consider intra aortic balloon pump and cardiac consult.
INTENSIVE INSULIN INFUSION
Introduction
-
The role of intensive insulin treatment is to maintain tight control of blood glucose in critically ill patients.
-
In a prospective, randomised, controlled study of mechanically ventilated adults, intensive insulin therapy reduced mortality to 4.6% compared with a conventional treatment group which had a mortality rate of 8%. The greatest reduction in mortality involved deaths due to multi-organ failure with a proven septic focus.
-
You may choose to use either Protocol A or Protocol B
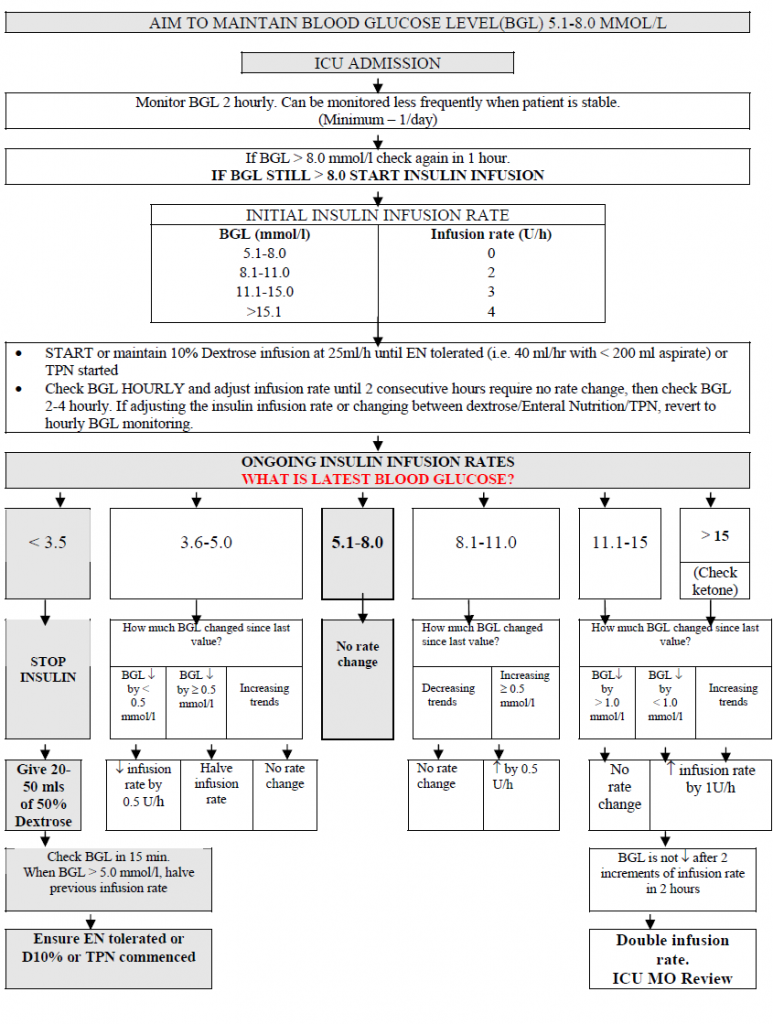
Protocol A
-
This protocol is not suitable for patients with diabetic ketoacidosis or children under the age of 16 years old.
-
The aim of blood glucose level (BGL) is between 5.1 – 8.0 mmol/l.
-
Start protocol when BGL exceeds 8.0 mmol/l after 2 consecutive readings in 2 hours.
-
Insulin Infusion: Use Soluble Insulin 50 units in 50 ml 0.9%NaCl infused through a dedicated cannula or central line lumen.
-
Blood glucose monitoring: Initially hourly monitoring, then 2-4 hourly when there is no rate change in 2 consecutive hours.
-
Patients who develop symptoms of severe hypoglycaemia should be treated as if BGL < 3.5 mmol/l. Symptoms include tremors, tachycardia, sweating, confusion and agitation leading to fitting and coma.
-
Feeding: Continuous feeding is recommended. Give IV dextrose 10% at 25ml/h until EN is tolerated or TPN is started. If EN is discontinued for any reason, recommence IV Dextrose 10% infusion at 25ml/h and continue insulin infusion.
-
Stop protocol when patient is taking food orally.
-
Other infusions (especially antibiotics) should be made up with water or saline if possible.
-
Patients should be converted to a standard hospital intermittent regimen (if required), before ICU discharge.
INTENSIVE INSULIN INFUSION IN THE ICU
Protocol B
-
Intensive insulin therapy is recommended to maintain serum glucose levels between 5 to 8 mmol/l in all ICU patients.
-
Continuous intravenous insulin infusion (CIVII) through a pump is preferred as it offers smooth control.
-
Dilute 50 units of soluble insulin in 50 ml of normal saline in a syringe and deliver it by an infusion pump.
-
Start CIVII with scale 1 or 2 initially.
-
Blood glucose level (BGL) should be monitored at 2 h intervals. Depending on whether the blood glucose improves 4 h later, the sliding scale may be switched to one with a higher initial CIVII rates. (e.g. from scale 1 to scale 3)
-
BGL may be monitored less regularly (i.e. 4 h intervals) once stable.
Continuous intravenous insulin infusion
| Blood
glucose (mmol/l) |
Scale 1
(U/h) |
Scale 2 | Scale 3 | Scale 4 | Scale 5 | Scale 6 | Scale 7 | Scale 8 |
| ≥ 22 | 3.0 | 4.0 | 5.0 | 6.0 | 7.0 | 8.0 | 10.0 | 11.0 |
| 18- | 2.5 | 3.5 | 4.0 | 5.0 | 6.0 | 6.0 | 8.0 | 9.0 |
| 14- | 2.0 | 3.0 | 3.0 | 4.0 | 5.0 | 5.0 | 6.0 | 7.0 |
| 12- | 1.5 | 2.5 | 2.5 | 3.0 | 4.0 | 4.0 | 4.0 | 5.0 |
| 10- | 1.0 | 2.0 | 2.0 | 2.0 | 3.0 | 3.0 | 3.0 | 4.0 |
| 8- | 1.0 | 1.5 | 1.5 | 1.5 | 2.0 | 2.0 | 2.5 | 3.0 |
| 6- | 0.5 | 1.0 | 1.0 | 1.0 | 1.5 | 1.5 | 2.0 | 2.0 |
| 5- | 0.5 | 0.5 | 0.5 | 0.5 | 1.0 | 1.0 | 1.5 | 1.5 |
| < 5 | Stop IV insulin infusion and inform doctor | |||||||
LUNG PROTECTIVE STRATEGY
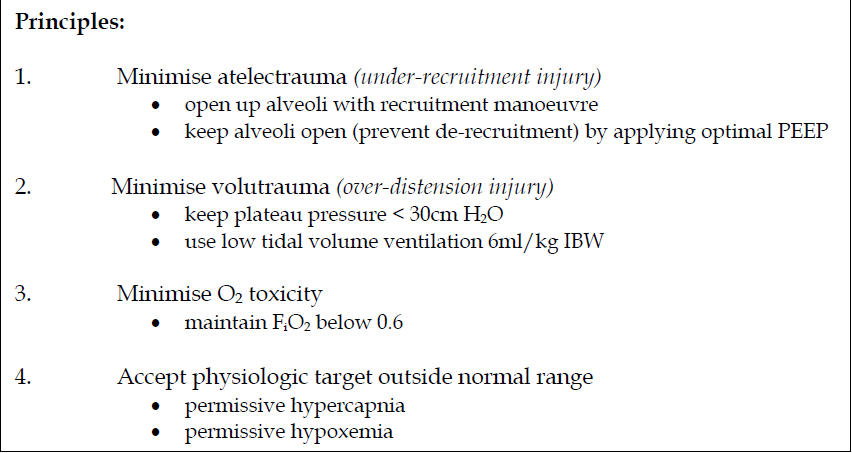
Recruitment manoeuvre
The patient must have relatively stable haemodynamics before a recruitment manoeuvre.
Choose one of the 2 options:
A. Step-wise incremental PEEP
-
Patient may need to be paralysed during the manoeuvre
-
Mode of ventilation: Pressure-controlled ventilation
-
Driving pressure (inspiratory pressure) : 15 cm H2O (up to 20 cm H2O)
-
Respiratory rate 10 – 12/min; I:E ratio 1:1; FiO2 1.0
-
Begin with PEEP 15 cm H2O. Ventilate for 5 – 6 breaths. Increase
PEEP by 5 cm H2O to 20 cm H2O. Ventilate for 5 – 6 breaths.
Reduce PEEP back to 15 cm H2O and ventilate for 5 – 6 breaths.
-
Repeat the process by increasing PEEP of 5 cm H2O (PEEP 25 cm H2O, then 30 cm H2O and 35cm H2O) each time till a peak pressure of 50 cm H2O is achieved.
-
At the end of the recruitment manoeuvre, perform an ABG with the following ventilatory settings PCV driving pressure 15 cm H2O, RR 10/min, I:E 1:2, PEEP 20 cm H2O and FiO2 1.0. PaO2 + PaCO2 > 400 mmHg suggests that there is less than 5% of the alveoli which are still collapsed.
-
If unable to achieve the above, repeat the recruitment manoeuvre to a higher peak pressure (driving pressure + PEEP) e.g. 55 cm H2O or 60 cm H2O or even higher while maintaining the driving pressure at 15 cm H20.
-
If still unsuccessful, consider repeating the recruitment manoeuvre in the prone position.
-
Repeat recruitment manoeuvre after position change, circuit break, or deterioration of lung mechanics or PaO2.
-
Monitor haemodynamics during manoeuvre. Terminate manoeuvre prematurely if:
-
SpO2 <85%
-
MAP < 60
-
HR < 60 or > increase more than 20% from baseline
-
New arrhythmia
-
B. PCV with PEEP method
-
Sedate and paralyse the patient
-
Pressure control ventilation
-
FiO2 1.0
-
I : E 1 : 1
-
Rate 8-10/min
-
PEEP 20 cm H2O
-
The peak inspiratory pressure is slowly increased to 50 – 55 cm H2O.
-
Ventilation at this peak pressure for about 2 minutes, after which the peak inspiratory pressure is lowered to 30-35 cm H2O.
C. PCV with PEEP method
-
Sedate and paralyse the patient
-
Pressure control ventilation
-
FiO2 1.0
-
I : E 1 : 1
-
Rate 8-10/min
-
PEEP 20 cm H2O
-
The peak inspiratory pressure is slowly increased to 50 – 55 cm H2O.
-
Ventilation at this peak pressure for about 2 minutes, after which the peak inspiratory pressure is lowered to 30-35 cm H2O.
Low tidal volume ventilation
-
Calculate ideal body weight (IBW) of the patient.
Male = 50 + 0.91 [height (cm) – 152.4]
Female = 45.5 + 0.91 [height (cm) – 152.4]
-
Mode: Pressure-controlled ventilation (preferred) or volumecontrolled ventilation.
-
Aim for tidal volume of 6ml/kg IBW while not exceeding plateau pressure (Pplat) of 30 cm H2O. In PCV, plateau airway pressure is equivalent to peak airway pressure. If volume-controlled ventilation is used, the plateau pressure needs to be measured regularly e.g. 2 – 4 hourly.
If Pplat > 30 cm H2O, decrease tidal volume by 1 ml/kg steps to 5 ml/kg or if necessary to 4 ml/kg IBW.
If breath stacking or severe dyspnoea occurs, tidal volume may be increased (not required) to 7 or 8 ml/kg IBW if Pplat remains ≤ 30 cm H2O.
-
Oxygenation: Aim for PaO2 55 – 80 mmHg or SpO2 88-95%
-
Arterial pH: aim > 7.1
The respiratory rate may be increased to a maximum of 35 /min. nfusion of intravenous NaHCO3 8.4% at 10 – 20 ml/hr may be considered.
Algorithm on lung recruitment manoeuvre using the step-wise incremental PEEP method
STRESS RELATED MUCOSAL DISEASE (SRMD) PROPHYLAXIS IN THE INTENSIVE CARE UNIT
Prophylaxis is routinely provided for critically ill patients admitted to the intensive care unit (ICU) who are at high risk for stress – related mucosal disease (SRMD), an erosive process of the gastroduodenal mucosa associated with abnormally high physiological demands. SRMDs are previously known as stress ulcers.
Not all ICU patients have the same risk of developing SRMD. Specific risk factors include :
-
mechanical ventilation ( more than 48 hours)
-
coagulopathy
-
shock states ( septic, haemorrhagic, cardiogenic, anaphylactic)
-
severe head injury and neurosurgical patients
-
severe burns ( more than 30%)
-
multiple organ failure
Patients in the ICU with history of gastric or duodenal ulceration, or with cirrhosis or acute renal failure, may benefit from prophylactic measures. Patients with multiple risk factors have an additive effect on the probability of developing SRMD.
Prophylactic therapy for SRMD
Considering available evidence and cost-effectiveness of current pharmacological agents for prophylaxis, the following are recommended:
-
IV Ranitidine 50 mg 8 hourly. Reduce dose to 50 mg 12 hourly in patients with renal failure.
The superior efficacy of intravenous H2 antagonists compared with sucralfate in preventing SRMD has been demonstrated, and therefore, H2 antagonists are preferred.
-
The use of proton pump inhibitors (PPI) as prophylaxis has not been shown to be superior to H2 antagonists and should probably be limited to those with history of recent UGIB or recent endoscopically proven ulcer.
IV Omeprazole or IV Pantoprazole 40 mg daily
PPIs are not renally eliminated and thus dose adjustment in renal impairment is not necessary
Treating clinically significant upper GI bleed in ICU
PPIs are the main stay of treatment in patients that develop clinically important UGIB usually given as infusion 8 mg /hr over 48 hours, as adjunct to endoscopic or surgical management.
Discontinuing SRMD prophylaxis
-
For those who do not have UGIB, prophylactic therapy is discontinued once patient is on full feeds and none of the above risk factors are present.
-
Consider changing to oral therapy as soon as tolerating orally.
-
For those who develop clinically significant bleed in ICU, PPIs are continued for at least 2 weeks (IV / oral Omeprazole or Pantoprazole 40 mg BD )
-
Although the potential protective effect of enteral nutrition on the gastric mucosa means that it should be considered as an adjunct to pharmacological prophylaxis in in appropriate cases, there is currently no evidence that enteral nutrition alone is sufficient to reduce the risk of stress related bleeding. Combination with pharmacotherapy has been shown to reduce SRMD incidence.
Risk of nosocomial pneumonia
No direct association has been found between the use of acid-suppressive therapy and nosocomial pneumonia. Factors other than elevated gastric pH probably contribute to pneumonia in the critically ill.
VENOUS THROMBOEMBOLISM PROPHYLAXIS RECOMMENDATIONS
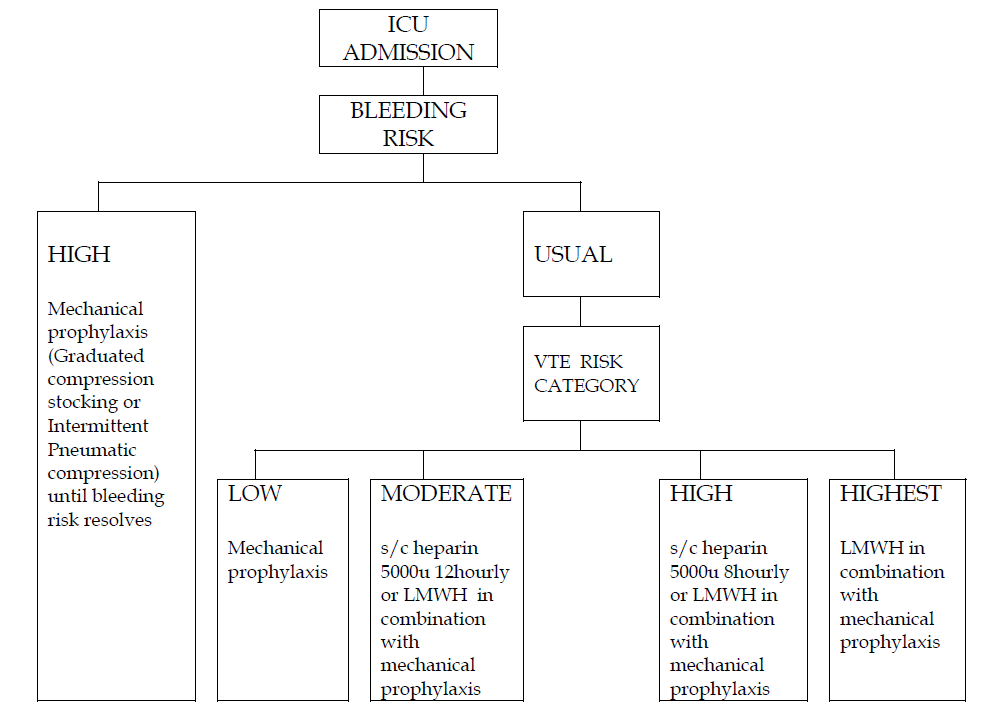
1) On admission to the intensive care unit (ICU), all patients should be assessed for their risk of venous thromboembolism (VTE). Accordingly, most patients should receive thromboprophylaxis. For patients who are at high risk of bleeding (eg. upper GIT bleeding, liver laceration etc.) use mechanical prophylaxis.
2) Consider withholding the heparin product when there is a significant decrease of platelet count (30 to 50% of initial count) or decrease to less than 100,000 per micro liter of blood or when INR > 1.5.
3) The prevention of VTE in neurosurgical has favoured mechanical prophylaxis methods. However the use of heparin products is considered to be safe after 48 to 72 hours.
4) Combined pharmacologic and mechanical prophylaxis may provide greater protection than either alone (e.g. s/c Heparin / low molecular weight heparin (LMWH) in combination with thigh-length antiembolism stockings or intermittent pneumatic compression).
5) Prophylaxis should generally not be interrupted for procedures or surgery unless there is a particularly high bleeding risk.
6) The insertion and removal of epidural catheters should coincide with the nadir of the anticoagulant effect. The last dose of LMWH should be 12 hours prior to removal of catheter and can be restarted 2 hours later.
7) Routine screening of patients for asymptomatic deep vein thrombosis is not recommended since this strategy is neither effective nor cost-effective.
8) At the time of discharge from the ICU, further thromboprophylaxis recommendations should be included in the transfer orders.
9) LMWH have a number of potential advantages over Low Dose unfractionated Heparin (LDUH) which include once daily administration, greater bioavailability, lower incidence of heparin-induced thrombocytopenia and cost effective due to less laboratory monitoring.
10) Early ambulation remains the most important non pharmacologic approach to prevention of VTE.
11) Prophylaxis should be reviewed daily and changed, if necessary.
Pharmacological modalities:
-
Low Dose unfractionated heparin (LDUH)
-
Subcutaneous (s/c) Heparin 5,000 units 8 hourly (high risk) or 12 hourly (moderate risk)
-
Low molecular weight Heparin (LMWH)
-
S/C Enoxaparin (Clexane) 40mg daily when creatinine clearance less than 30ml/min or 30mg daily when creatinine clearance greater than 30ml/min
ABSOLUTE RISK FOR VTE
Patient category |
Recommendation |
Low riskeg. medical patients, immobilization, use of pharmacologic paralysis or sedation, heart failure |
Mechanical prophylaxis |
Moderate riskeg. general surgery, major gynecologic surgery, major urologic surgery, sepsis, vasopressor use,active medical condition |
LMWH or s/c Heparin 5000units 12 hourly in combination with mechanical prophylaxis |
High riskeg. stroke, neurosurgery, previous VTE |
LMWH or s/c Heparin 5000 units 8 hourly in combination with mechanical prophylaxis |
Highest riskeg. spinal cord injury, major trauma hip/knee arthroplasty, hip fracture surgery |
LMWH in combination with mechanical prophylaxis |
ALGORITHM FOR VTE PROPHYLAXIS IN ICU
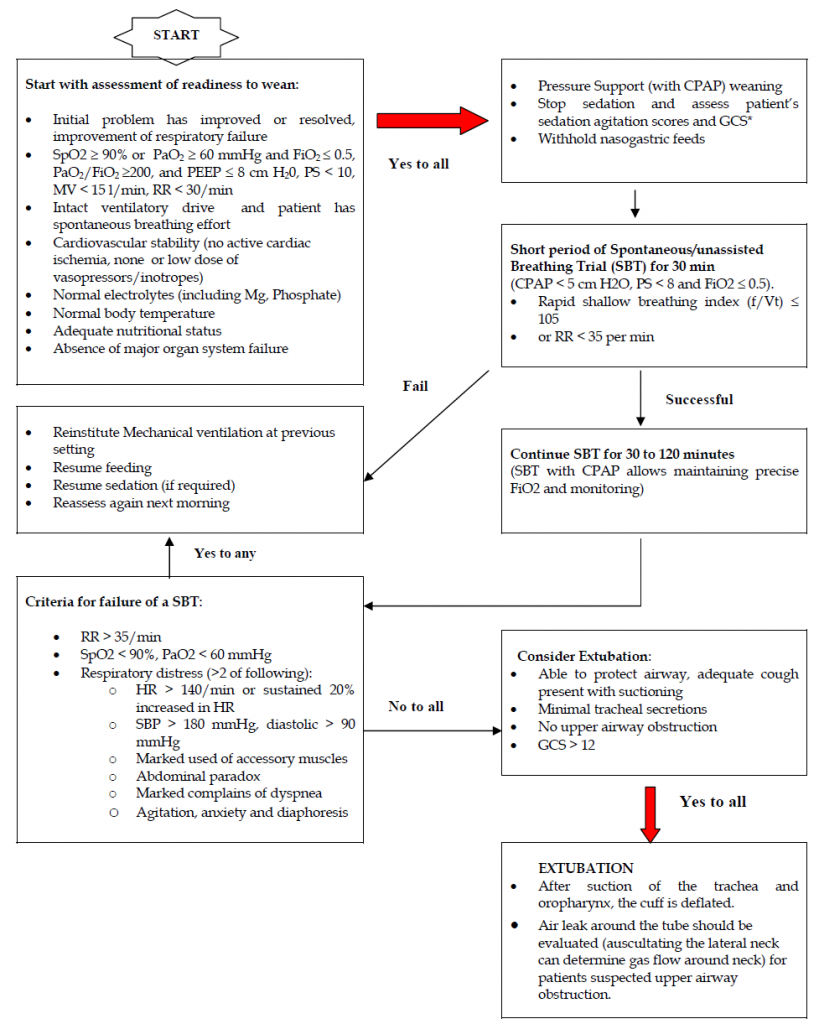
WEANING OR DISCONTINUATION FROM MECHANICAL VENTILATION
In patients requiring mechanical ventilation for more than 24 hours, a search for all causes that may be contributing to ventilator dependence should be undertaken. Reversing all possible ventilatory and non-ventilatory issues should be an integral part of the weaning process.
Before a patient is considered a candidate for discontinuation of ventilator support, a basic level of physiologic readiness must be established.
Refer to the flow chart.
Common reasons for weaning failure:
-
Chronic hypercapnic state
-
Decreased CNS drive
-
Weaning to exhaustion and inadequate rest of respiratory muscles
-
Reduced respiratory pump capacity
-
Poor nutritional status
-
Electrolyte disturbances (decreased calcium, magnesium and phosphate levels
-
Polyneuropathy of critical illness
-
Corticosteroid therapy
-
Prolonged neuromuscular blockade
-
-
Auto-Peep
-
Increased airway resistance (bronchospasm, endotracheal tube obstruction)
-
Decreased lung or chest wall compliance
-
High ventilation requirements due to increased dead space
-
Overfeeding
-
Myocardial ischaemia, left heart failure
-
Infection/fever
-
Major organ system failure
Points to remember:
-
Ensure an appropriate mix of work and rest periods, assurance of proper sleep and nutrition are helpful.
-
Patients who fail a spontaneous breathing trial (SBT) should have the cause for the failed SBT determined. Once reversible causes for failure are corrected, subsequent SBT should be performed every 24 hours.
-
Patients who fail an SBT should receive a stable, non-fatiguing, comfortable form of ventilatory support.
-
Protocols to decrease the use of continuous intravenous sedation reduce the duration of weaning.
-
Tracheostomy should be considered when it is apparent that the patient requires prolonged ventilator assistance. Even though the timing of tracheostomy is still controversial, it is recommended to consider tracheostomy if anticipated ventilation is going to be > 7 days. Advantages of tracheostomy include decrease need for sedation, more secure airway enabling greater patient mobility, improved efficiency of airway suctioning, faster weaning from mechanical ventilation and reduced length of stay in the ICU.
-
Unless there is evidence for clearly irreversible disease (e.g. high spinal cord injury, advanced amyotrophic lateral sclerosis), a patient requiring prolonged mechanical ventilatory support for respiratory failure should not be considered permanently ventilator dependent until 3 months of failed weaning attempts.
-
Weaning strategy in the prolonged mechanically ventilated patient should be slow-paced, and include gradually lengthening spontaneous breathing trials.
Ref: Management Protocols in ICU, Malaysian Society of Anaesthesiologists